Exploring the Impact of the New European Directive on the Pharmaceutical Industry - Clinical Trials Arena

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library

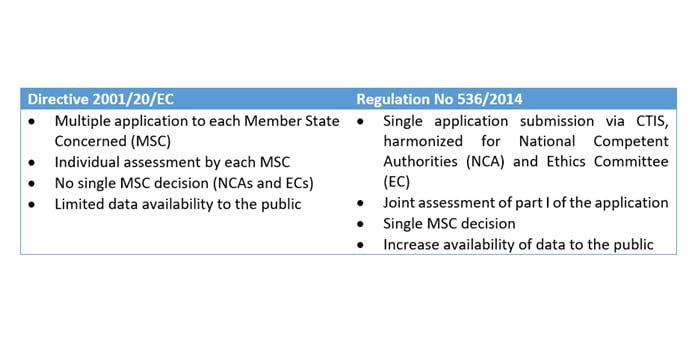
EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download

Book 6: 2023 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC

Buy The Pocket Guide to the EU Directives for Clinical Research: Clinical Trial Directive 2001/20/EC, GCP Directive 2005/28/EC, GMP Directive 2003/94/ EC Book Online at Low Prices in India | The Pocket Guide
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials