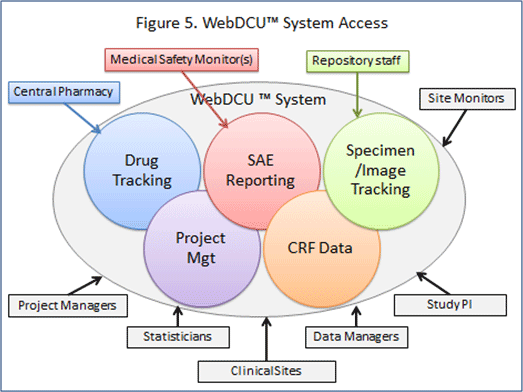
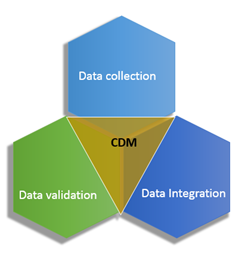
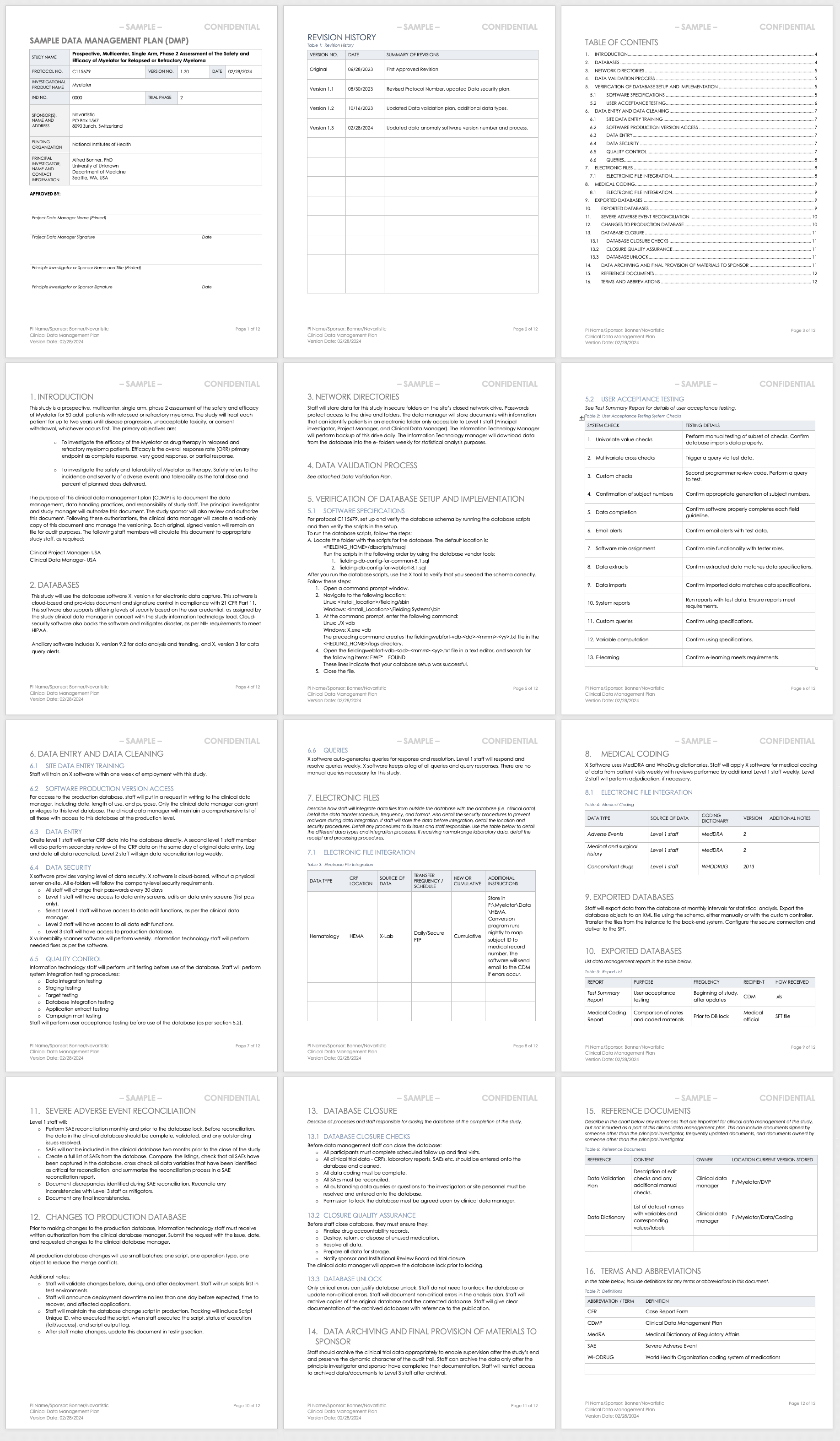
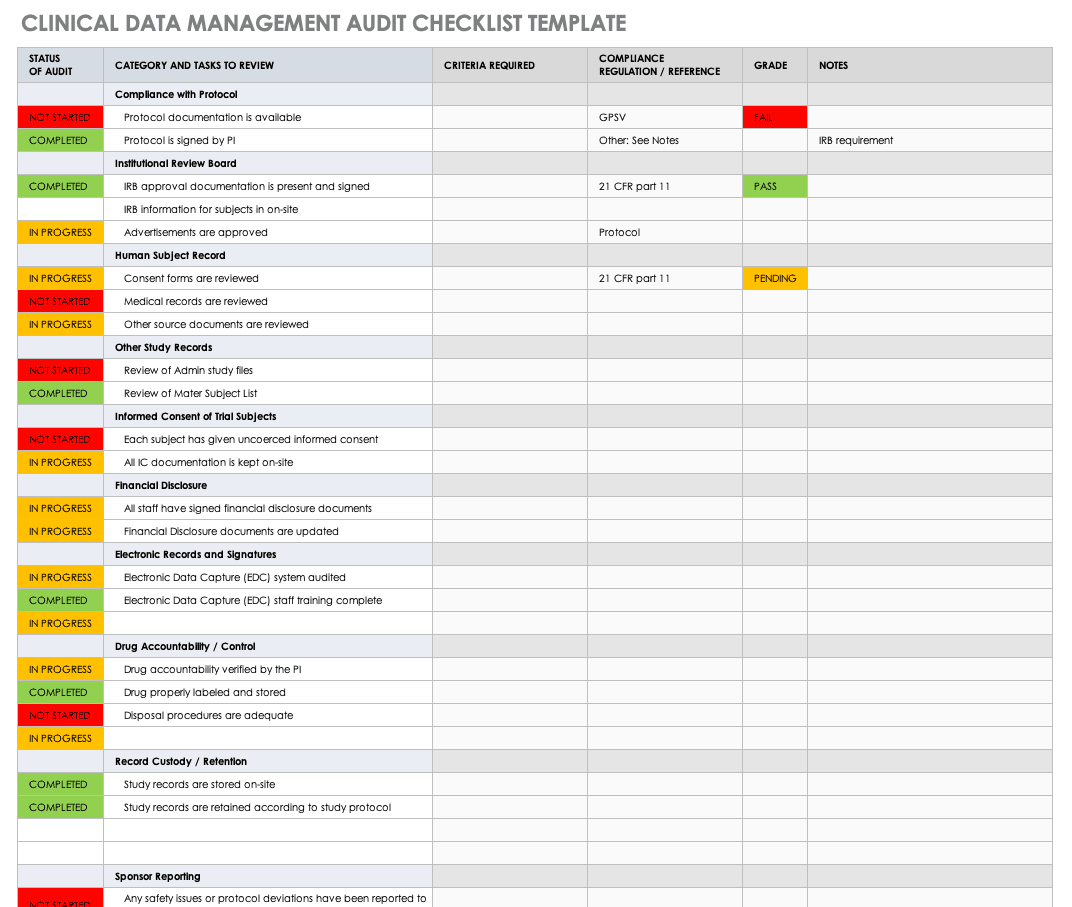
PDF) A Model Data Management Plan Standard Operating Procedure: Results From the DIA Clinical Data Management Community, Committee on Clinical Data Management Plan

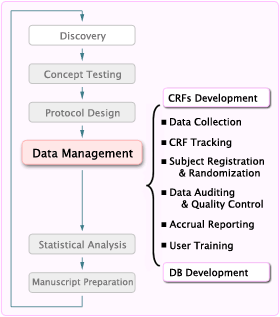
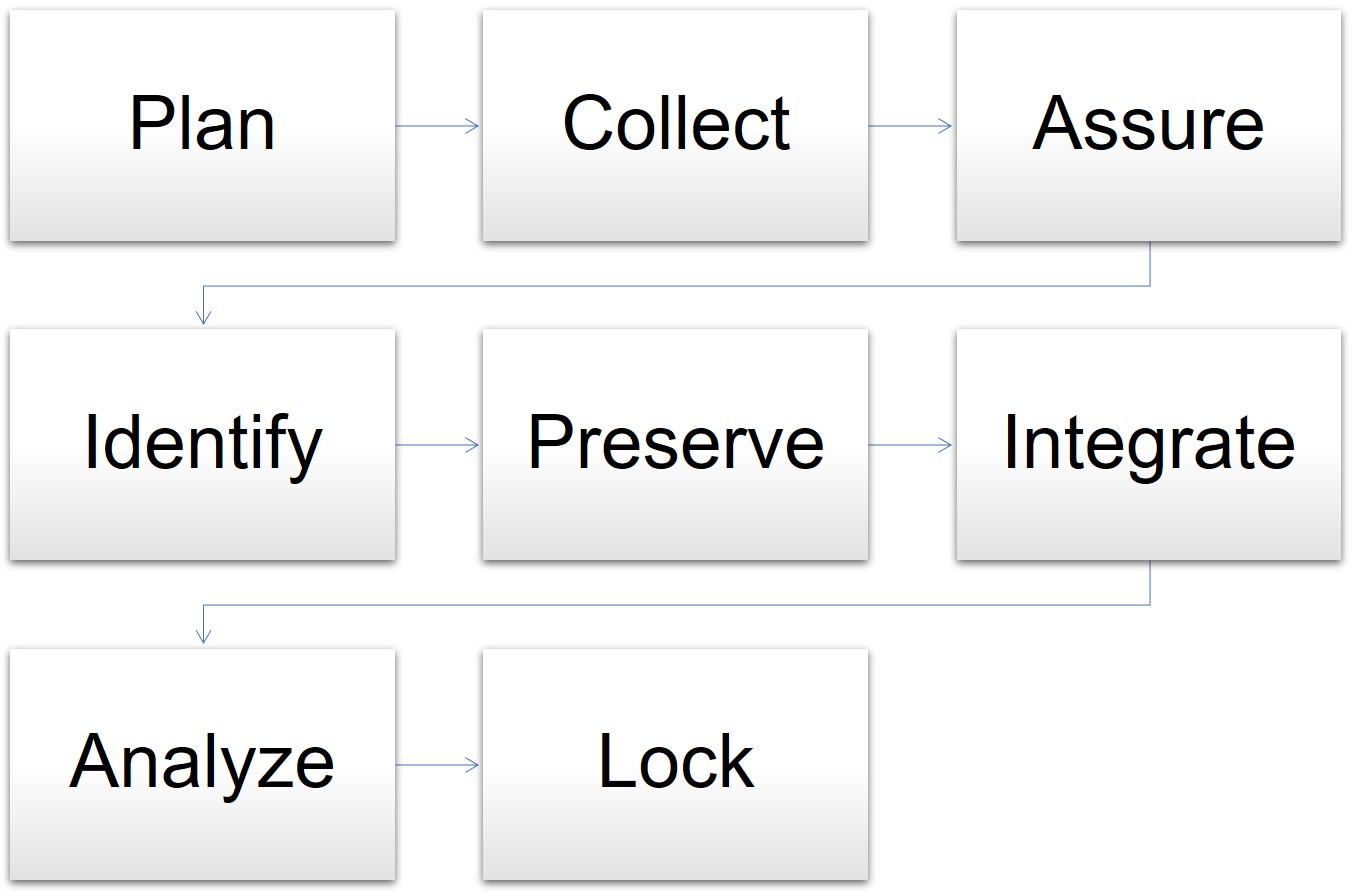
Data Management in Clinical Trials. E-CRF design / P-CRF Data entryData validation Data import Clinical coding DATABASE CLOSURE Database Closure Documents. - ppt download