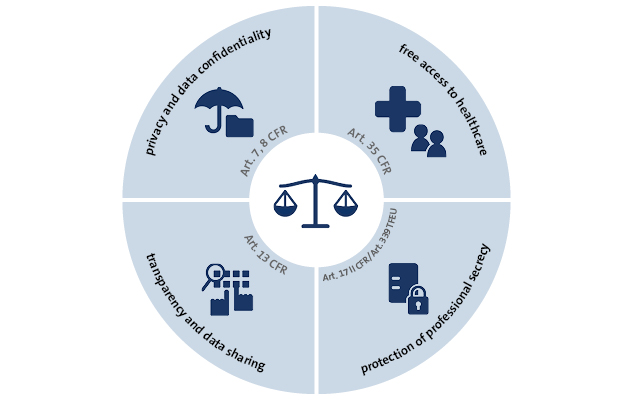
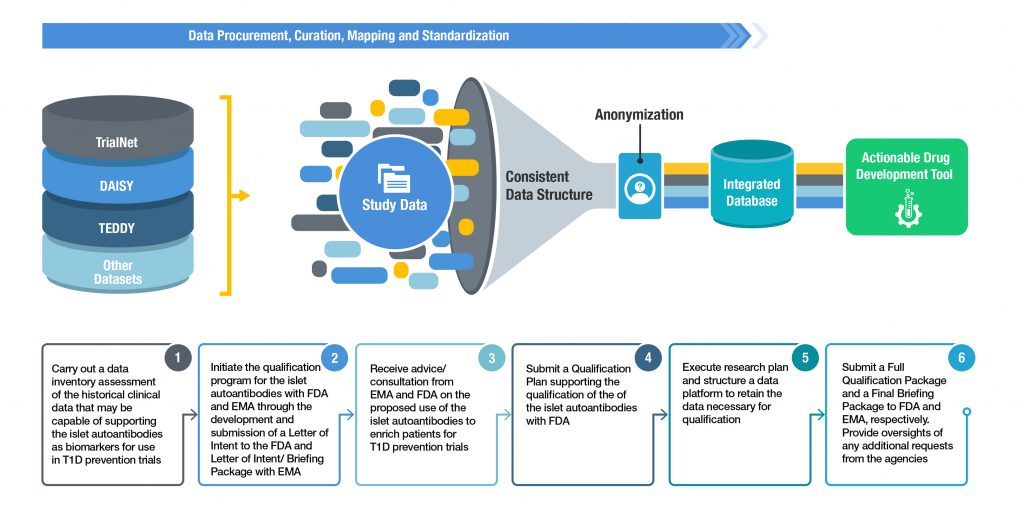
Comparative overview of Preclinical Data studies and Clinical Trials... | Download Scientific Diagram

Call to pool research resources into large multi-centre, multi-arm clinical trials to generate sound evidence on COVID-19 treatments | European Medicines Agency

EMA: Points to consider on implications of COVID-19 on methodological aspects of ongoing clinical trials - Meditrial Helpline

Assessment of the Regulatory Dialogue Between Pharmaceutical Companies and the European Medicines Agency on the Choice of Noninferiority Margins - Clinical Therapeutics
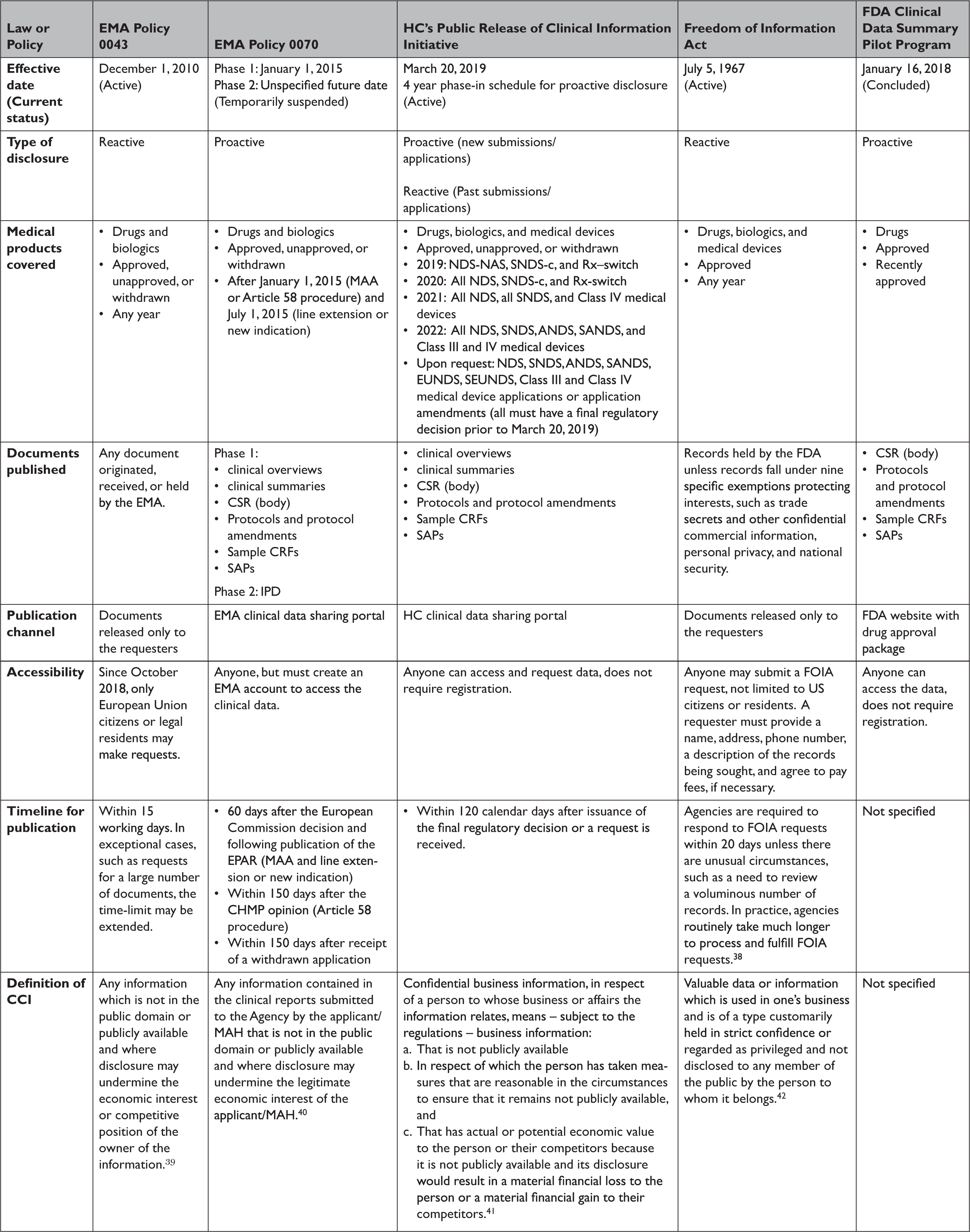
Transparency of Regulatory Data across the European Medicines Agency, Health Canada, and US Food and Drug Administration | Journal of Law, Medicine & Ethics | Cambridge Core

Clément Provansal on LinkedIn: Regulatory harmonisation of clinical trials in the EU: Clinical Trials

Positive outcome for ema's policy 0070, one year after the publication of the clinical trial results - Portolano Cavallo

Clinical Trial Regulation Update - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety