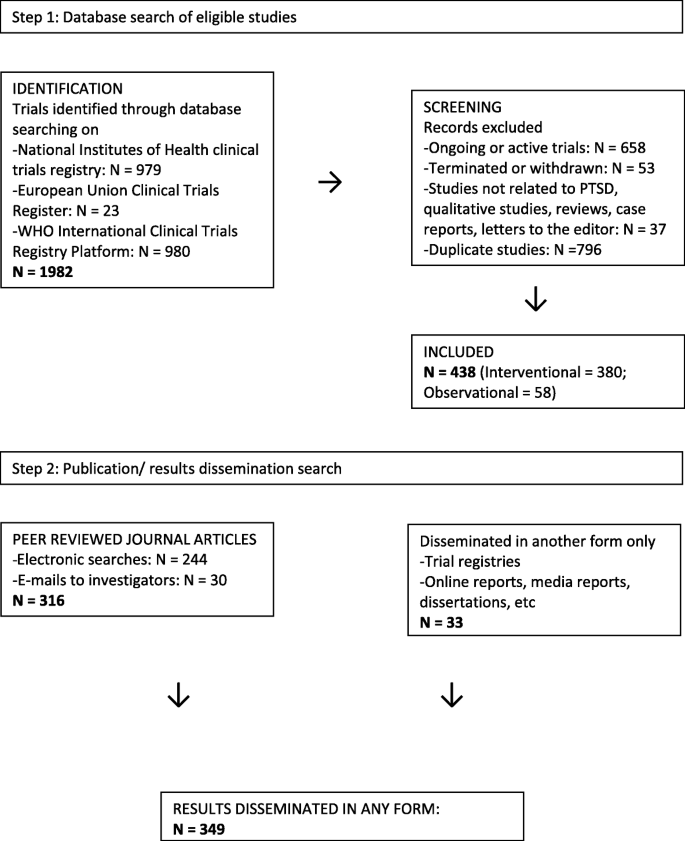
Flow of diagram of study. Abbreviation: eU-cTr, european Union clinical... | Download Scientific Diagram

Clinical trial success relies on effective patient recruitment – International Clinical Trials Day 2022 | ECRIN
What Are the Documents Required for Clinical Trial Applications to Regulatory Authorities in Europe? - Sofpromed

New regulation on clinical trials in Spain - Leon Research | CRO - Clinical Trials Spain, Italy and Portugal

Flow of diagram of study. Abbreviation: eU-cTr, european Union clinical... | Download Scientific Diagram

Prevalence of clinical trial status discrepancies: A cross-sectional study of 10,492 trials registered on both ClinicalTrials.gov and the European Union Clinical Trials Register. | Bennett Institute for Applied Data Science