EMA and FDA psychiatric drug trial guidelines: assessment of guideline development and trial design recommendations | Epidemiology and Psychiatric Sciences | Cambridge Core
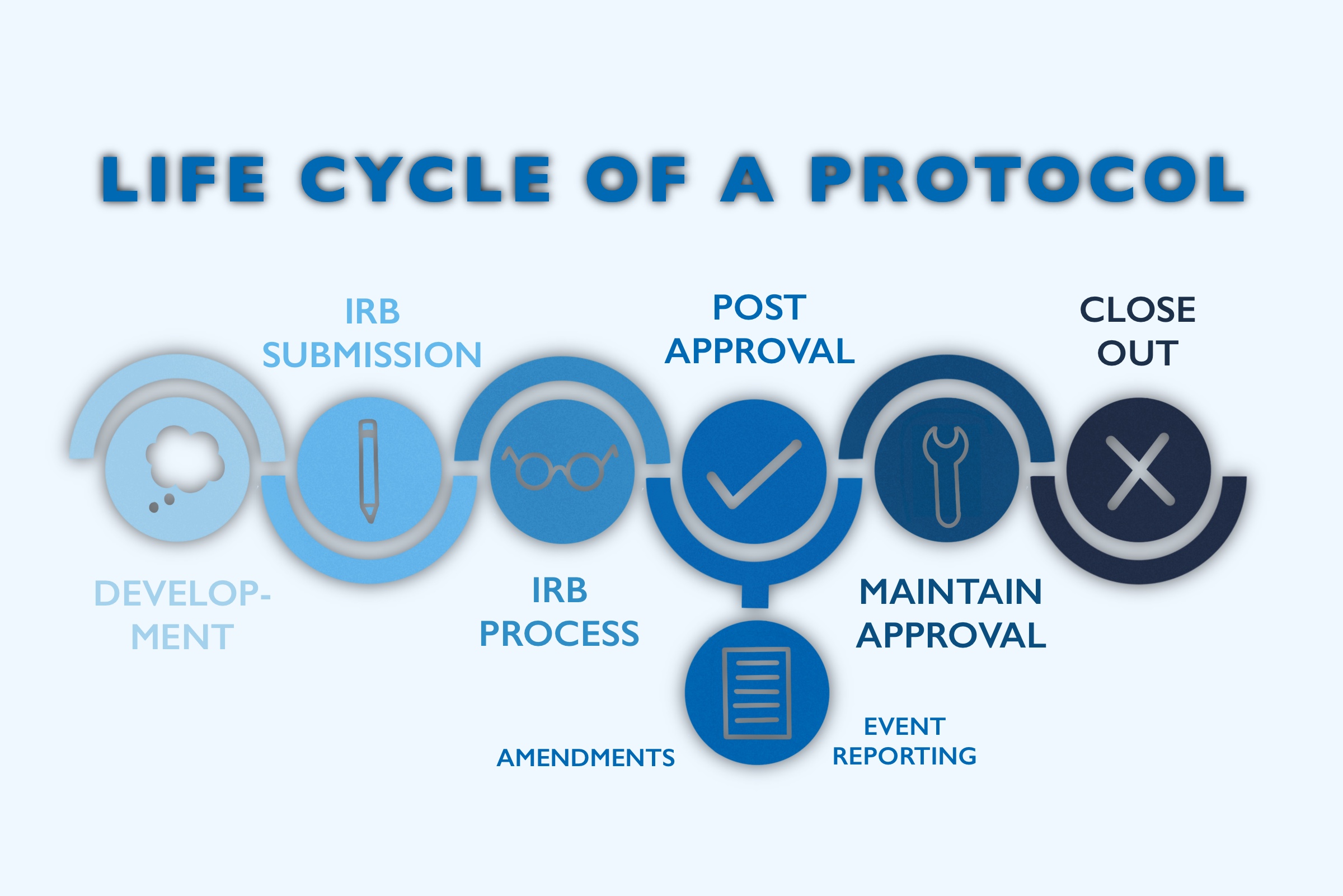
How do Institutional Review Boards (IRB) and Ethics Committees (EC) impact clinical trials? - Clincierge

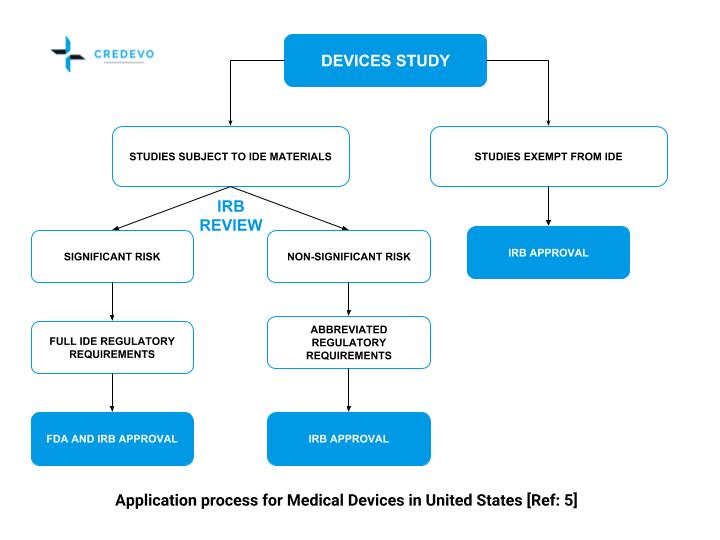
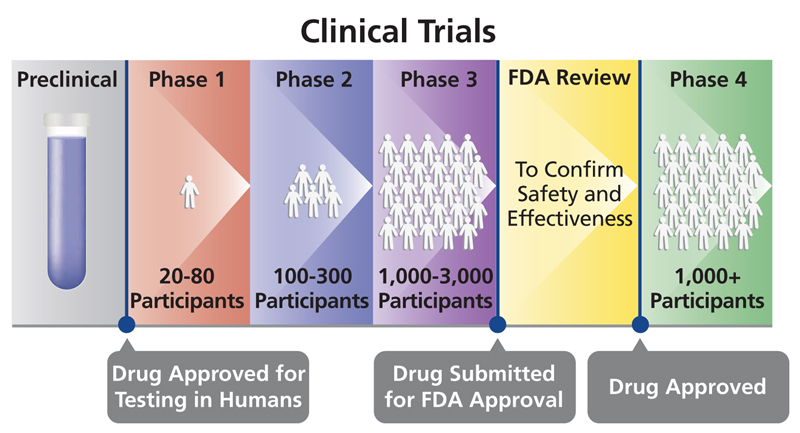
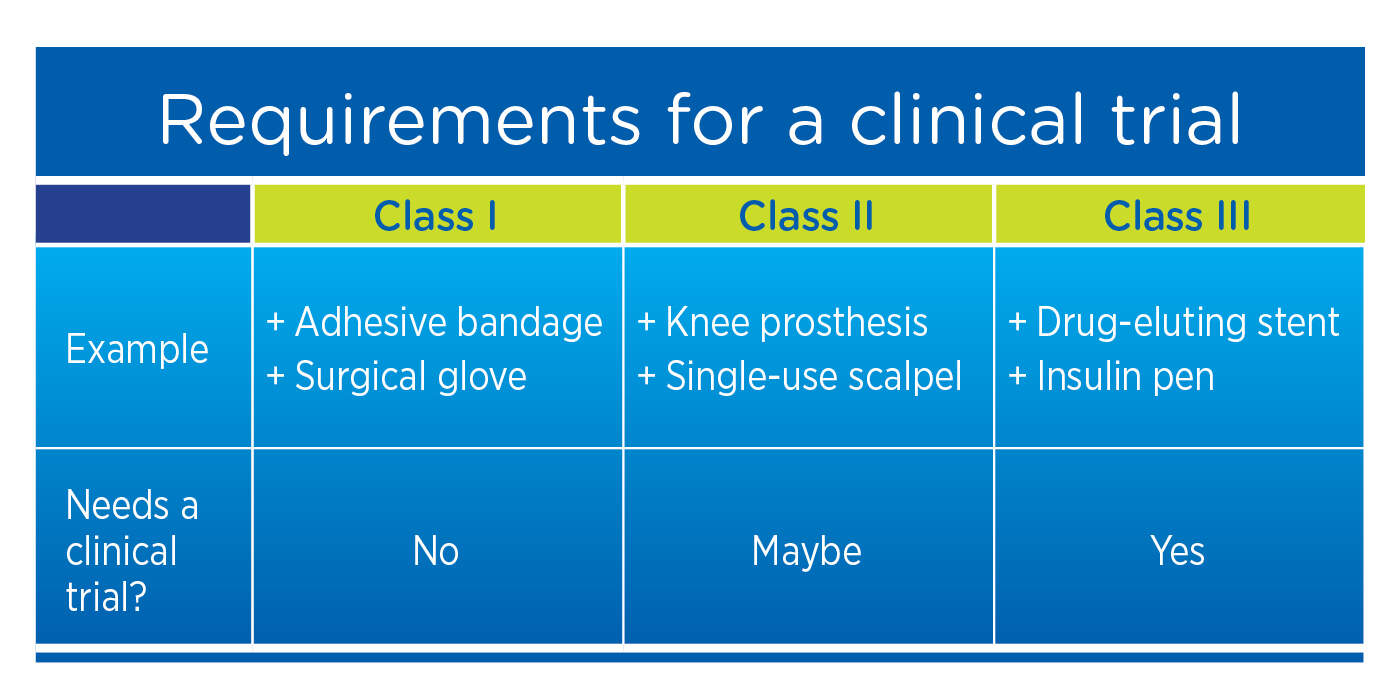
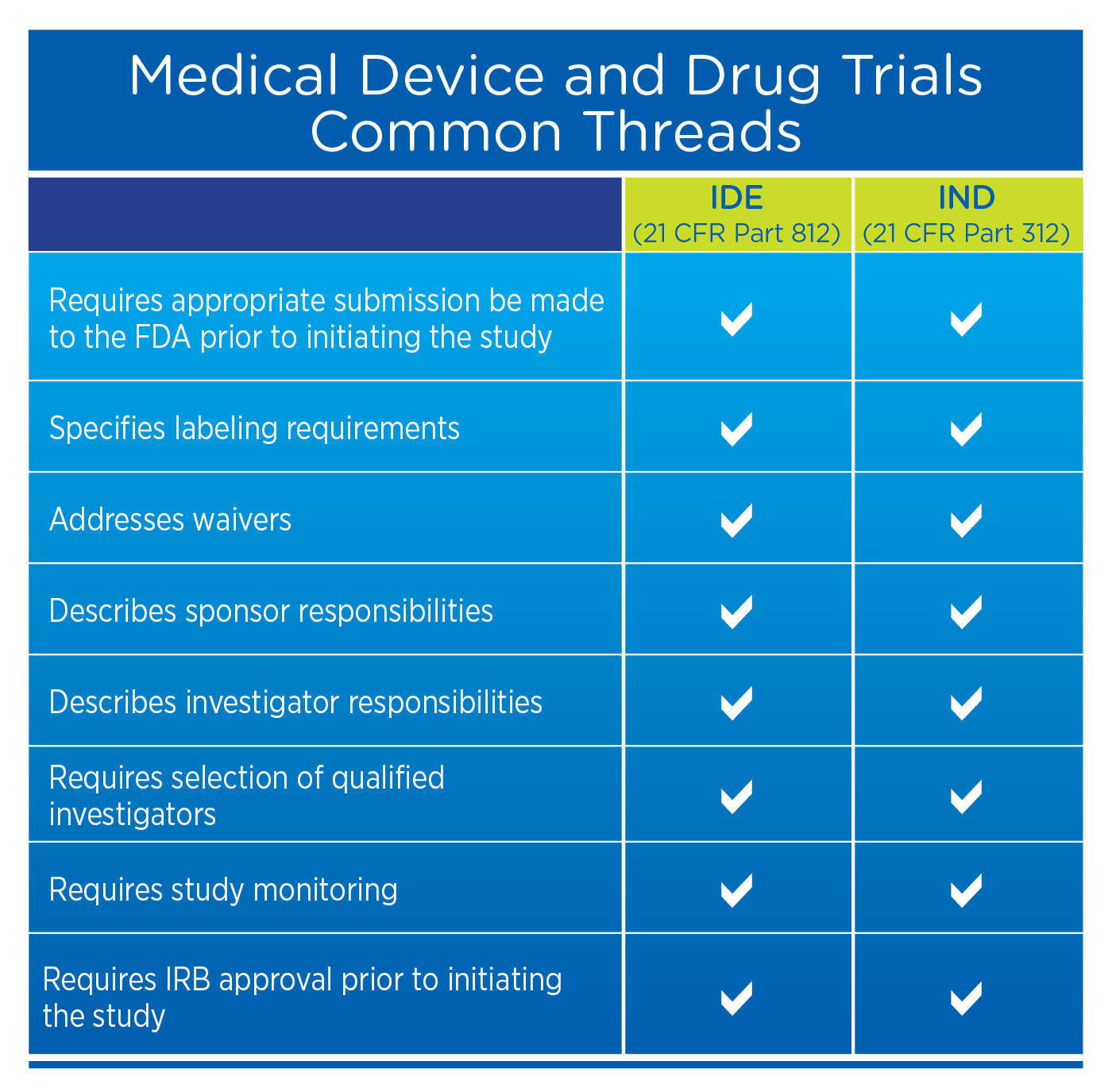
Regulation of Medical Devices and their Clinical Trials Studies in the USA: An Update | Bentham Science

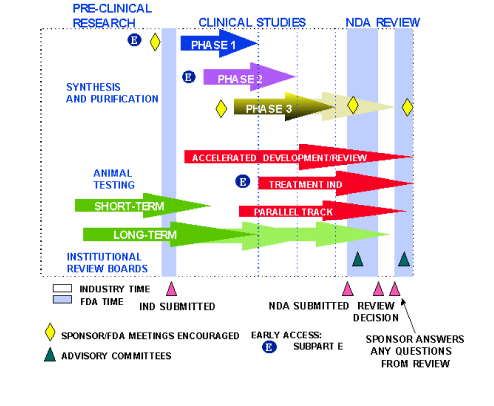
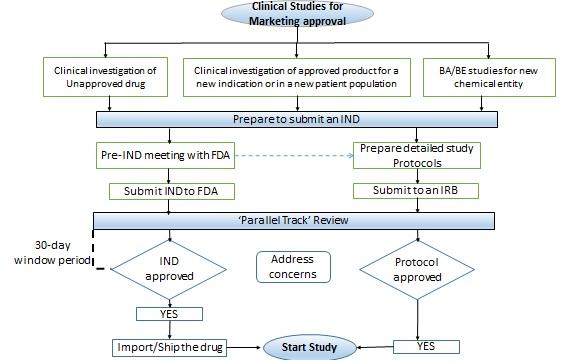
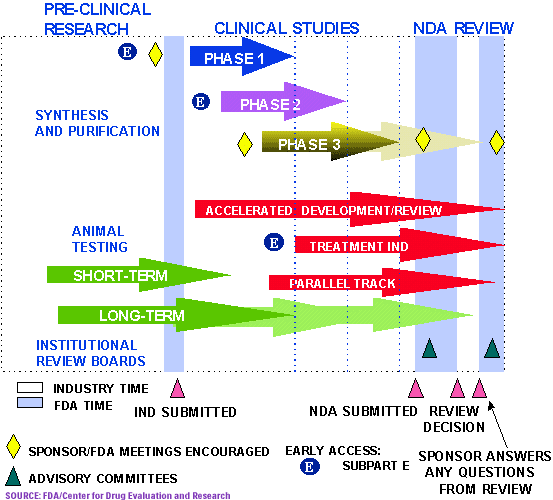
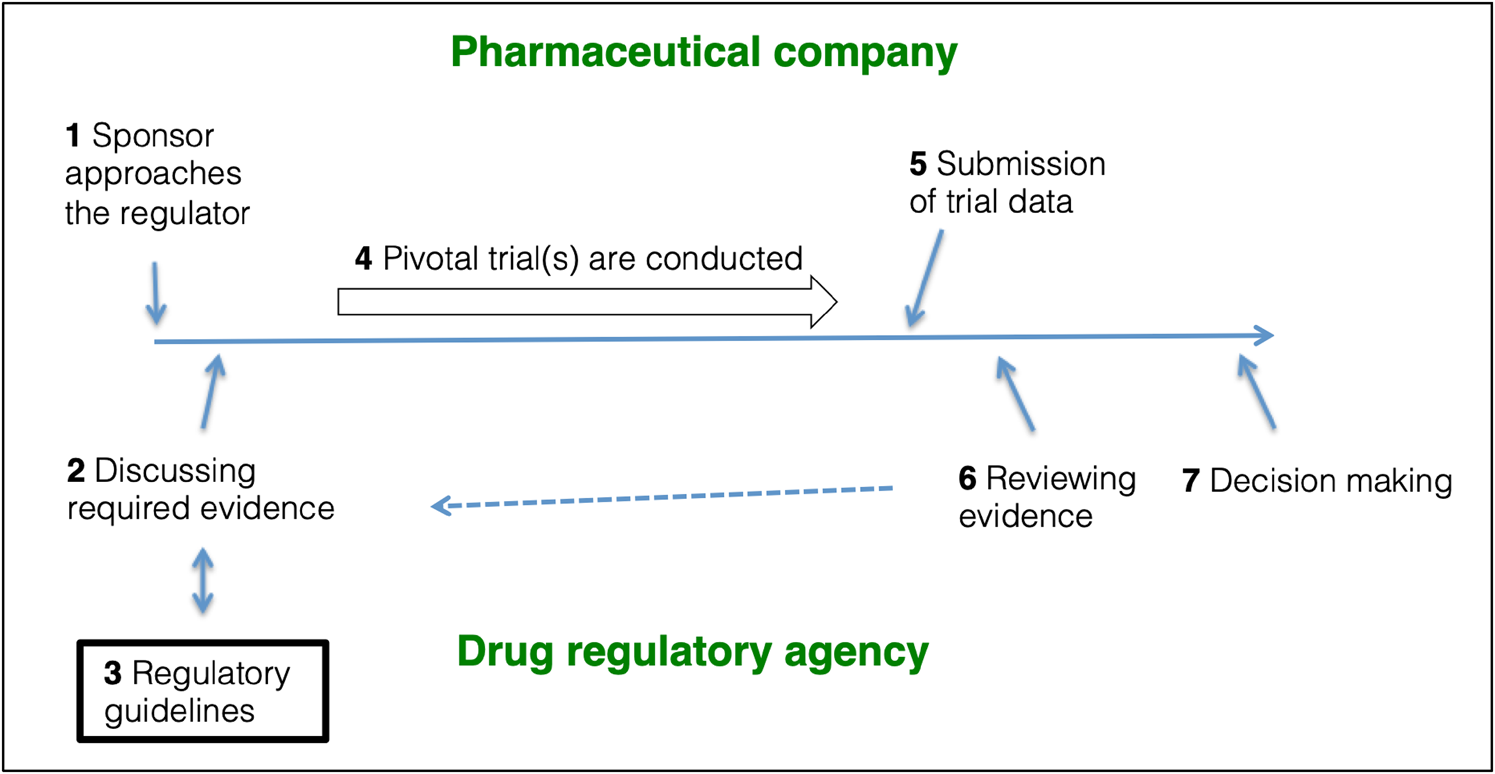
Rare Disease Research in the United States: Understanding FDA Guidance and Flexibility in the Application of Regulatory Standards · BioBuzz

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet